In this month’s blog post, Dr William Johnston, a research assistant in Biological and Biomedical Sciences and a member of the SHIP team, outlines how biofilms are changing microbiology research.
I have been involved in microbiology research since back in my undergraduate days here at GCU, where I was focussing on catheter-related bloodstream infections. After graduating in 2018, I was accepted for a PhD position at the University of Glasgow to research gum-disease, and have since returned to GCU as a research assistant to investigate novel therapeutics for bacterial vaginosis. What links all of these conditions, and many more, is the development and formation of microbial ‘biofilms’ during the onset of disease.

Although biofilms were originally described in the 17th century by Antonie van Leeuwenhoek, their role in disease development has only been appreciated much more recently. Biofilms form when microbes (bacteria and fungi) adhere to surfaces and produce a slime-like matrix. Being encapsulated within this slime, bacteria are able to tolerate much higher doses of antibiotic and antimicrobial therapies, allowing them to survive and drive further disease. Currently, it is thought that most bacteria exist as biofilms in nature despite their free-floating ‘planktonic’ form being the most well-studied in conventional microbiology research.
As our understanding of biofilms has evolved, it has been found that many – if not the majority! – of human infections are caused by these peculiar slime bugs, particularly chronic and recurrent conditions. As such, many researchers have developed methods for studying these biofilms in the laboratory. My research at GCU focusses both on investigating novel anti-biofilm therapies, and developing models to accurately test the efficacy of these treatments. Biofilms are seldom composed of one type of bacteria or fungi, and exist largely as mixed communities. Therefore, it is really important that we capture this complexity in our testing so we can mimic human infection as much as possible.
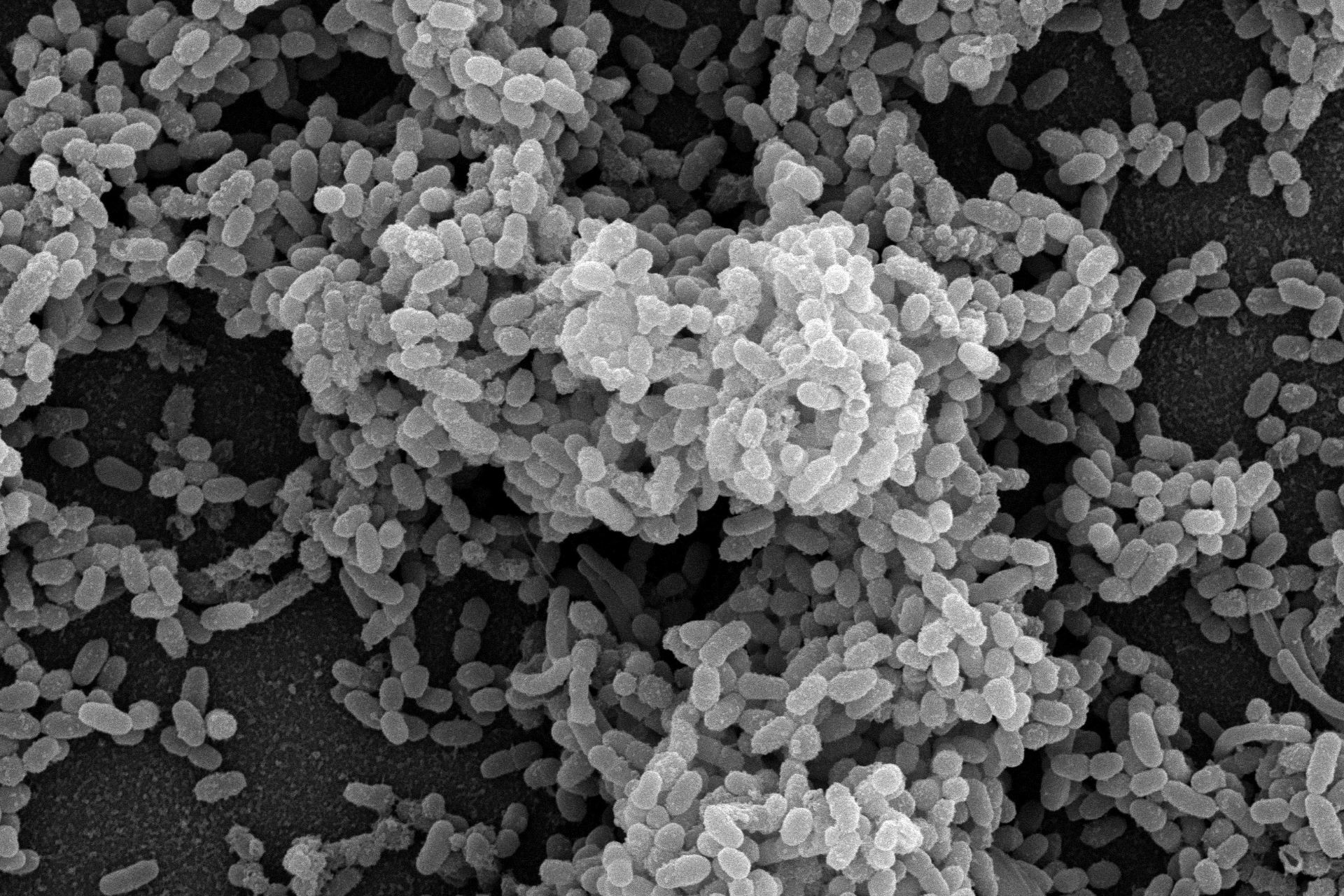
Moving forward it is my hope that we start to identify new ways to overcome and disrupt these slime layers, and start to integrate more biofilm testing in the preliminary stages of drug development. This is an important first step in looking at new treatments, so we can have higher confidence that they will work in real life settings. Understanding the features that can promote, inhibit and disrupt biofilms may be a crucial first step towards eventually achieving this goal!
To find out more about the SHIP team, head on to the GCU website, read the rest of our blogs and follow us on Twitter @SHIPGCU